
The number of individuals with diabetes is expected to exceed 690 million worldwide in the next 20 years. Diabetic kidney disease (DKD), defined as the coexistence of chronic kidney disease (CKD) and diabetes, affects nearly 35% of diabetic patients and accounts for up to 50% of end-stage kidney disease (ESKD) cases.
This project investigates how metabolic stress and mitochondrial dysfunction in diabetes activate innate immune pathways and drive proinflammatory cytokine production in glomerular cells. We integrate data from diabetic animal models with multiomics datasets from DKD patients obtained through the Kidney Precision Medicine Project.
The goal is to identify molecular subtypes that explain part of the heterogeneity in DKD mechanisms and to define patient groups that may benefit from targeted therapies.
Chronic Kidney Diseases (CKD) are common among African American patients. The excess risk for CKD in this population is largely explained by social determinants of health and by the presence of genetic variants in the APOL1 gene that are unique to African ancestral populations. The pathogenic mechanisms responsible for the genetic association remain poorly understood. Most importantly, the lack of consensus on the mechanisms by which APOL1 risk variants induce kidney diseases hinders the development of specific therapies.
Our major focus is to study glomerular and single-cell transcriptomes from kidneys and organ models to uncover transcriptional phenotypes associated with the presence of APOL1 kidney risk variants. Our goal is to extract gene signatures that could inform about the pathobiology of APOL1, and help to identify potential therapeutic agents.
I am an advocate for team science, and part of my research depends on collaborations with different teams of kidney investigators and NIH consortia. In particular, the Nephrotic Syndrome Study Network (NEPTUNE) and the Kidney Precision Medicine Project (KPMP). I also coordinate the Renal Chalk Talk series for the Cleveland Kidney Center, an initiative that promotes collaboration among kidney researchers and clinicians from Case Western Reserve University, Cleveland Clinic, University Hospitals, the Louis Stokes Cleveland VA Medical Center, MetroHealth, and Cleveland State University. Finally, I contribute to WikiPathways as a Community Editor, focusing on the area of Renal Genomics.
Research Strategy
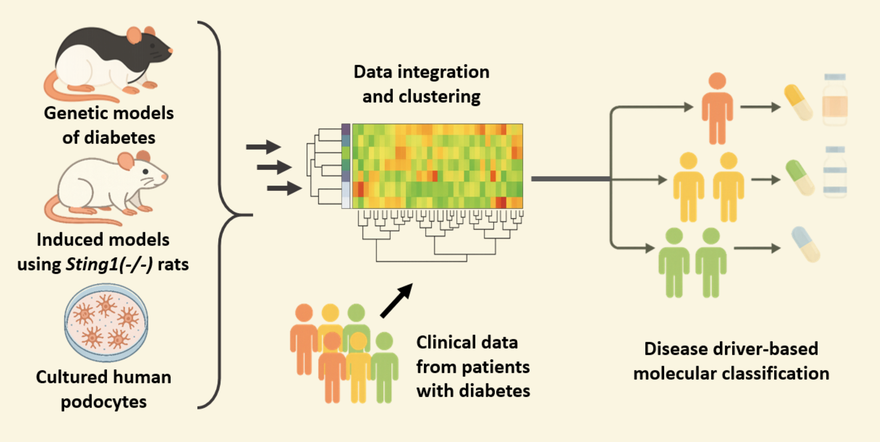
Our goal is to identify molecular subtypes that account for the heterogeneity of diabetic kidney disease (DKD) mechanisms and define patient groups that may benefit from targeted therapies. To achieve this, we integrate data from diabetic animal models with multiomics datasets from DKD patients generated by the Kidney Precision Medicine Project.
| Disciplines |
|---|
| Animal Models, Bioinformatics, Genomics, Metabolism, Systems Biology |
| Organ Systems |
| Renal System |
| Diseases |
| Chronic kidney disease, Diabetes, Hypertension |
| Major Techniques |
| Bulk RNA-Seq, Cell Culture, Cellular and Molecular Biology, Dietary Interventions, Gel Electrophoresis/Western Blots, In-vivo Animal Models, Quantitative RT-PCR, RNA Isolation and Gene Expression Analysis, Single-Cell/Nucleus RNA-Seq |
| Genes |
| APOL1 (Apolipoprotein L1), STING1 (None) |